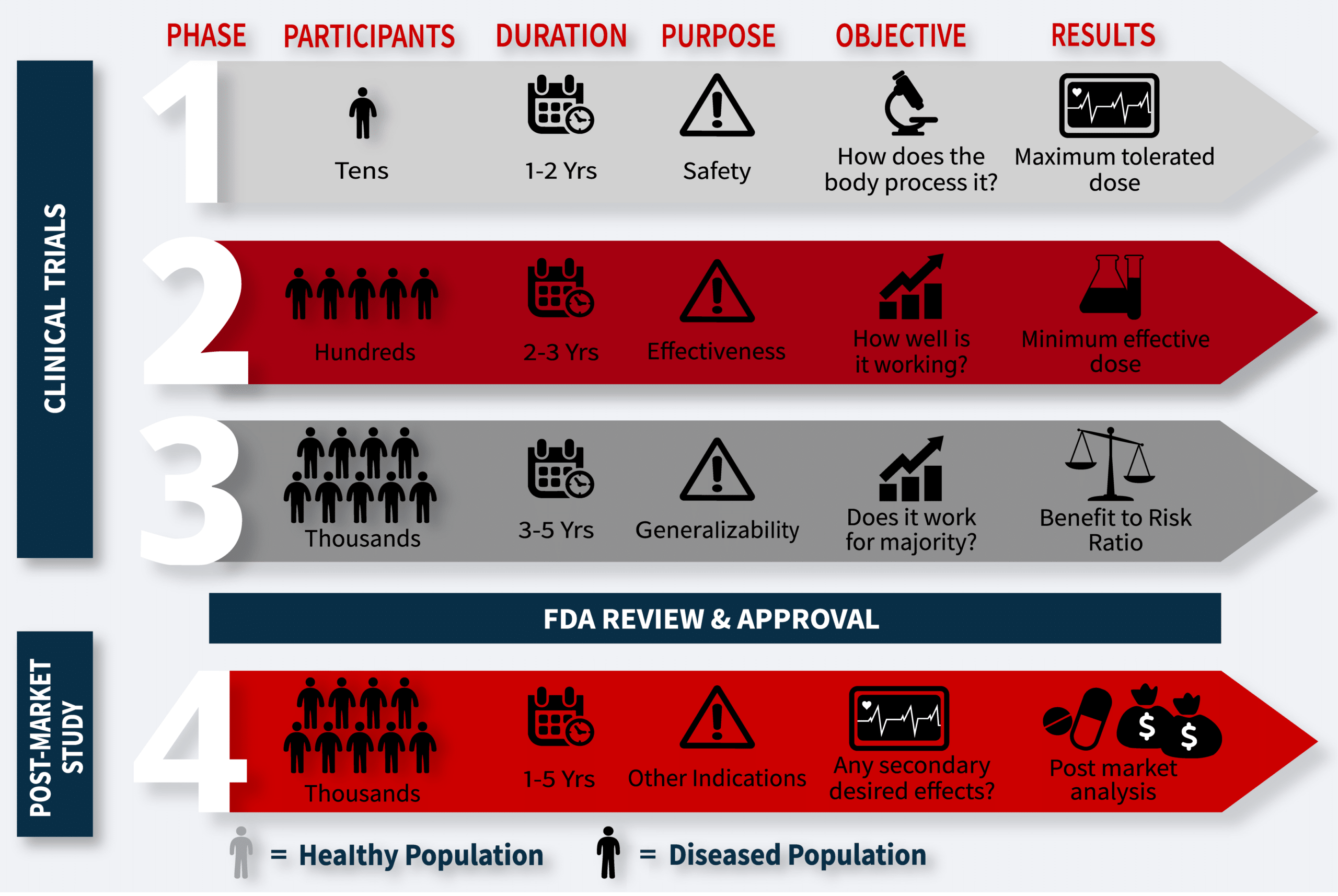
Clinical Trial Phases
Clinical trials unfold through stages known as phases, with each phase crafted to address a distinct research question.
Phase I
Researchers test a new drug or treatment in a small group of people for the first time to evaluate its safety, determine a safe dosage range, and identify any possible side effects.
Phase II
The drug or treatment is given to a larger group of people to see if it is effective and to evaluate its safety further.
Phase III
The drug or treatment is confirmed in a third phase to test its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug or treatment to be used safely.
Phase IV
Studies are done after the drug or treatment has been marketed to gather information on the drug’s effect in various populations and any side effects associated with long-term use.